Describe in Words How to Calculate an Empirical Formula
Steps to Calculate Empirical Formula of Hydrocarbon. Divide the number of moles of each element by the smallest number of moles.

Empirical Formula Definition Steps Examples Video Lesson Transcript Study Com
If the moles are all whole numbers then youre done and thats your empirical formula.
. Add up the atomic masses of the atoms in the empirical formula. Empirical Formula - A formula that gives the simplest whole-number ratio of atoms in a compound. X n m4 2.
If the mass per cent of various elements present in a compound is known its empirical formula can be determined. Number of moles Mass given in grams Molar mass of element. The simplest ratio of H.
Molecular Formula vs Empirical Formula. 304g N 1 1 mol N 1401g N 217 mol N 217 1 mol N 696g O 1 1 mol O 1600g O 435 mol O 217 2 mol O-----Step 4. Calculate moles of CO2formed.
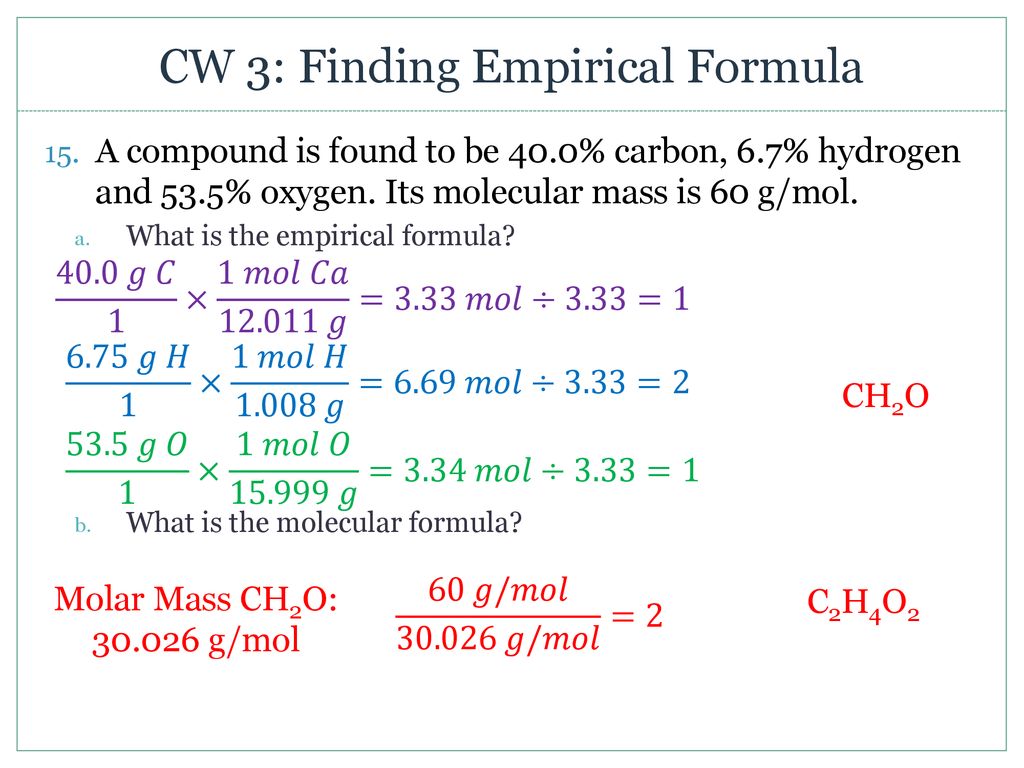
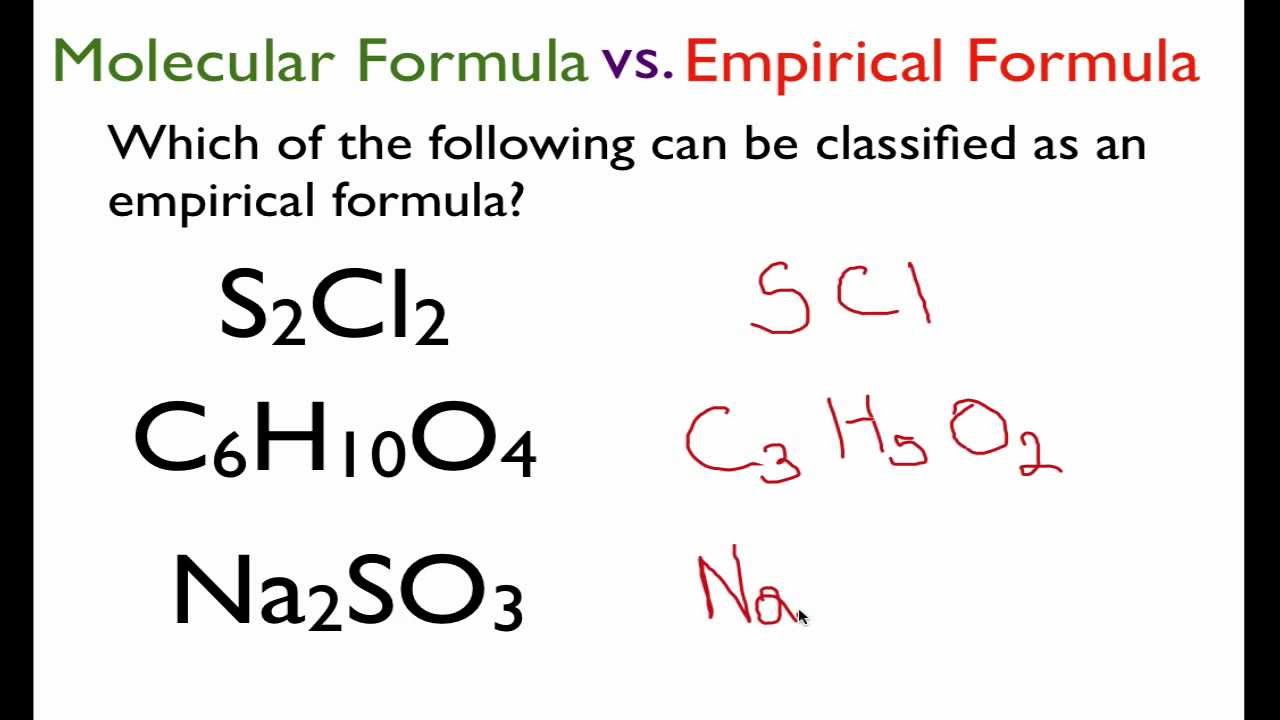
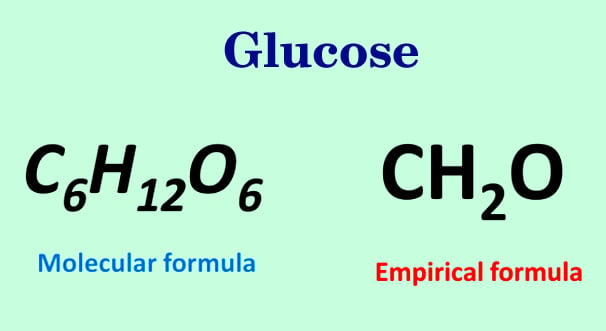
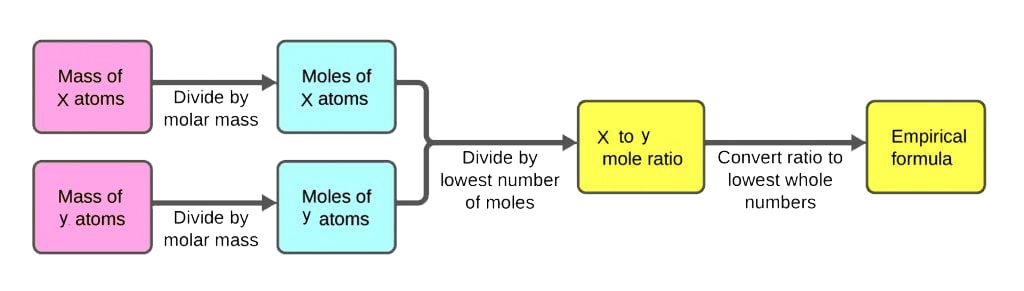
In chemistry the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound. An empirical formula represents the simplest whole number ratio of various atoms present in a compound whereas the molecular formula shows the exact number of different types of atoms present in a molecule of a compound. Start with the number of grams of each element given in the problem.
This video demonstrates an approach that can be used to calculate an empirical formula from mass dataOther Stoichiometry videosSandwich Stoichiometry - ht. However its usual to use the smallest whole number ratio of atoms. Steps for Determining an Empirical Formula 1.
From this formula we can say that our organic compound is vitamin C. Molecular formula can further. Calculate the mass of each element in grams.
Moles of CO2formed Moles of C in original hydrocarbon compound. The formula could be articulated as H1110O5549. Determine the number of moles by dividing the grams by the atomic mass.
For example the empirical formula of a hydrocarbon is CH 2 and its M r is 42. When n 1 it usually means that the empirical formula is the same as the molecular formula. Set up generic balanced equation for combustion of Hydrocarbon Cn.
The steps for determining a compounds empirical formula are as follows. Calculate the empirical formula mass. Change of each element into grams for example if the compound contains 40 carbon then change it to 40 g carbon Convert grams of each element into moles by dividing grams by molar mass.
Note just for interest. If we multiply all the subscripts in the empirical formula by 2 then our molecular formula will be. Hm CnHm x O2 nCO2 m2 H2O.
By dividing the lowest number alter the numbers to whole numbers. The empirical formula tells us the relative ratios of different atoms in a compound. Determine the masses of each component in the compound.
In that case the mean z-score is 0 and the standard deviation is 1. In our case if we know that the sample contains 40 C 67 H and 533 O we can plug these numbers into the calculator and get the same formula. To calculate the empirical rule you need to be provided with a mean and standard deviation for a bell-shaped normal distribution.
C 6 H 8 O 6. Plug the empirical mass and the molar mass into the boxed-up formula from earlier in this post. If you want to.
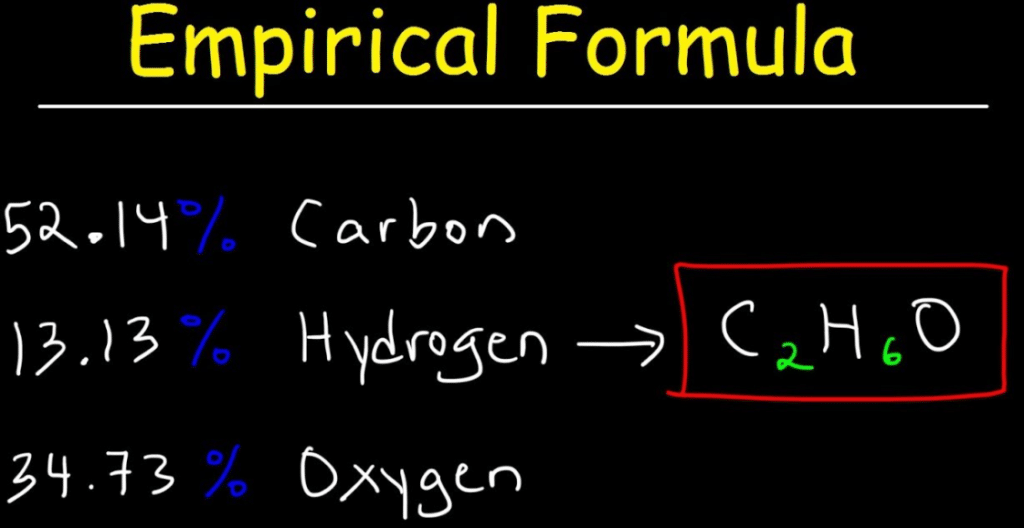
The steps are1 Write the atoms involved in the calculation2 Write the mas. To calculate the empirical formula we can also use percentages instead of masses in grams. Well learn how to calculate molecular formula for a compound when you are given its empirical formula and its molar mass.
Formula to calculate empirical formula. Element percentage mass in grams text m Step 2. In this JC1 webinar lets learn how to calculate empirical and molecular formula of ascorbic acid or Vitamin CThe empirical formula of a compound shows the.
H 1110 5549 2000. Also the online empirical formula calculator also uses the above formula to find the number of moles for a given composition of elements in a compound. Otherwise you can also use z-scores with the empirical rule.
Count the number of moles of each type of atom that is present. Notice that n can have values from 1 2 3 and so on. In order to do this you need to f.
O 5549 5549 1000. Steps for Finding The Empirical Formula Given Mass Percent. Where the molar mass of each element varies according to the presence of isotopes.
O If percentages are given assume that the total mass is 100 grams so that the mass of each element the percent given. Combine the moles of each atom into an empirical formula.
Empirical Formula Definition Get Education
Chemical Formulas Boundless Chemistry
Empirical Formula Calculator How To Find Empirical Formula Of A Compound

How To Calculate An Empirical Formula Chemistry Worksheets Chemistry Notes Chemistry Lessons

Empirical And Molecular Formula Chemistry Class 11 Some Basic Concepts Of Chemistry

Calculating Empirical Formula Chemistry Math Aqa

Unit 3 Holey Moley How Does The Mole Concept Illustrate Constant Composition And Conservation Of Mass How Can We Predict The Relative Amounts Of Substances Ppt Download

Empirical Rule Definition Formula Statistics By Jim

Determining An Empirical Formula From Percent Composition Data Worked Example Video Khan Academy
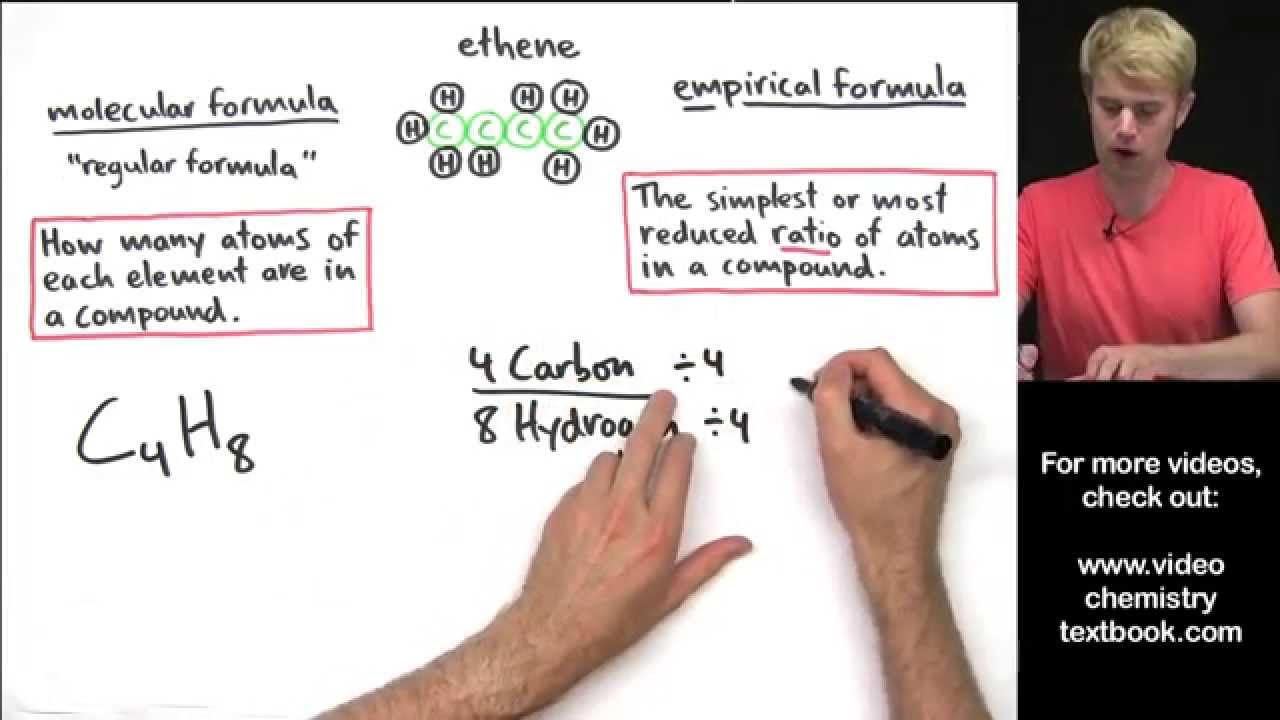
Empirical Formula And Molecular Formula Introduction Youtube
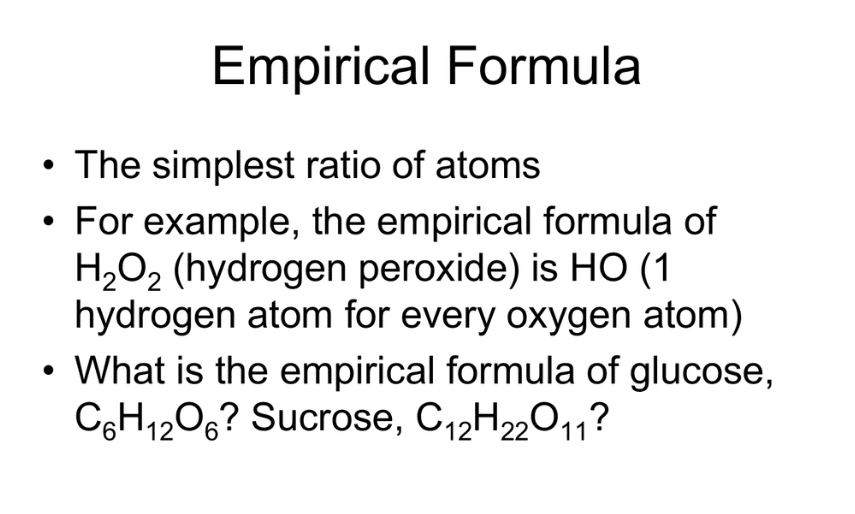
Empirical Formula Definition Get Education

Empirical Formula Definition Steps Examples Video Lesson Transcript Study Com

Percent Composition Chemistnate
What Does The Empirical Formula Of A Compound Describe Quora

How To Calculate An Empirical Formula Chemistry Worksheets Chemistry Notes Chemistry Lessons
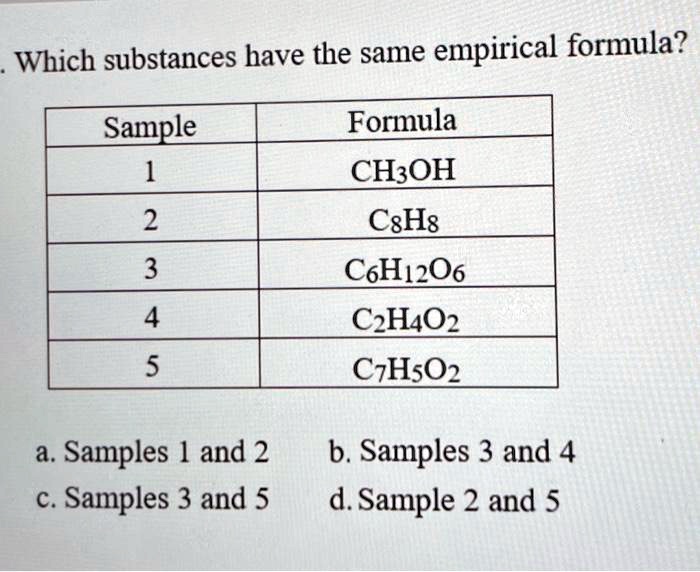
Solved Which Substances Have The Same Empirical Formula Sample Formula Ch Oh 2 Cxhs 3 Cohz06 4 Czh4oz 5 Czhsq2 A Samples 1 And 2 C Samples 3 And 5 B Samples 3 And D Sample 2 And 5
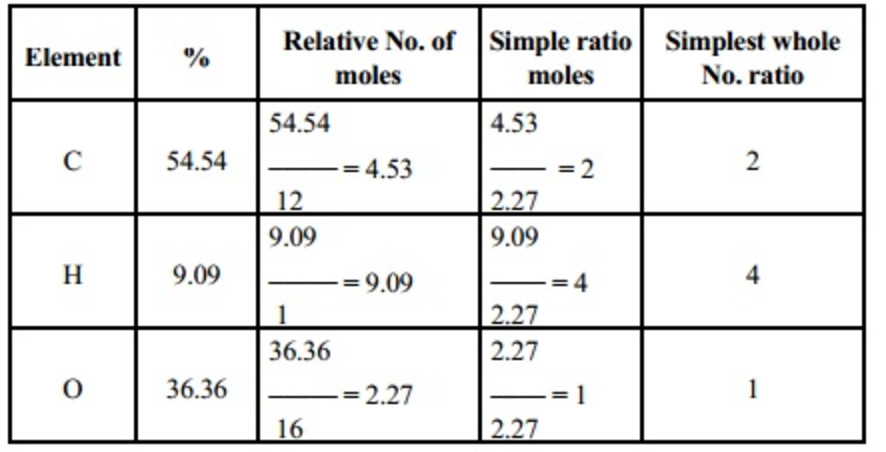
Empirical And Molecular Formula Chemistry Class 11 Some Basic Concepts Of Chemistry
Empirical Formula Calculator How To Find Empirical Formula Of A Compound
